
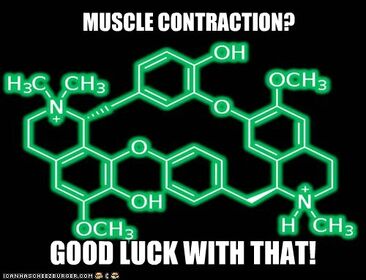
A molecule of d-tubocurarine in its protonated form. DTC is monoquaternary, but its second nitrogen can gain a hydrogen atom when it's in the body.
BPAGE UNDER CONSTRUCTION. DO NOT TRUST IT. DO NOT EDIT IT.
d-tubocurarine is awesome. It's also knwn as dTC.
Most of those those other puny nondepolarizing NMBs are just synthetic wannabe molecules made in laboratories. dTC, on the other hand, is the real thing. It's one of the active ingredients in freaking curare (the other being toxiferine). It was originally used to kill people and animals. That's what I'm talking about.
It is an alkaloid that comes from the plant Chondrodendron tomentosum, a vine native to South America, from the plant family Menispermaceae.
Plantae, Magnoliophyta, Magnoliopsida, Ranunculales, Menispermaceae, Chondrodendron tomentosum
It works by binding to acetylcholine receptors and preventing acetylcholine from binding to them. It can also apparently bind to a few other receptors such as GABA and 5-HT(3) receptors. This is because those receptors have very similar structures to ACh receptors. And you might ask, if it can bind to GABA receptors, why doesn't it cause tetanus or something? The answer is quite simple, GABA receptors are located in the brain, and dTC doesn't cross the blood-brain-barrier, so it never gets the chance. It primarily binds to and blocks nicotinic acetylcholine receptors at the neuromuscular junction, acetylcholine can't bind to receptors to trigger a depolarization and action potential within the skeletal muscles, and you don't move.
DTC is formed through chemical reactions involving dopamine and tyramine. You actually have the ingredients to make dTC floating around in your body right now. Be thankful that you don't actually have the enzymes that make it too. Just remember, every time you eat dairy that has tyrosine that your body will make into tyramine and dopamine, you're eating the ingredients to make dTC. Luckily, you actually can ingest dTC and live... (I don't suggest it; it tastes bad. Not that I've ever eaten it, but alkaloids tend to be bitter. Also, if you have any sort of cut or tiny opening on your mouth or gums or something and it gets into your bloodstream...)
It was first introduced into anesthesia on January 23, 1942 by Harold Griffith on a patient undergoing an appendectomy, and it was first used for killing people way before you were born. The plant itself existed millions of years before you were born. Its order, Ranunculales, is the oldest order of flowering eudicots.
The "d" in d-tubocurarine stants for "dextrorotatory." Sometimes, it can be called (+)-tubocurarine, which is just the same as saying "d-." There is such thing as "l" or "levorotatory" tubocurarine, but the "d" variant is more potent and studied and used more often. But now that they're trying to study and develop new NMB drugs, studying LTC more as a potential NMB or just to see what makes it less potent wouldn't be a bad idea... I mean atra's cis isomer actually eliminated histamine release. And even d- and l- isomers can be quite different from each other.
There are a few forms of it that one may come across when talking about dTC: a protonated and unprotonated form. The protonated form can exist when dTC is inside the human body, and the second nitrogen can become positively charged with its 3 original bonds to carbon atoms and a new 4th bond to a hydrogen. However, dTC is still seen as a monoquaternary bisbenzyltetrahydroisoquinoline with the chemical formula C37H41N2O6 when it is not protonated (H42 when it is). What I mean by monoquaternary is that it has one quaternary ammonium cation. A quaternary ammonium is a nitrogen bonded to four carbon atoms, and it has a positive charge (I call it the N+ of death). Something that is monoquaternary has one quaternary ammonium, and quaternary ammoniums are permanently charged. The other nitrogen that sometimes has a positive charge is technically not quaternary because not all four bonds are carbon, and the nitrogen is not positively charged if it's not protonated.
There is also a bisquaternary molecule similar to tubocurarine known as metocurine, and it is sometimes called dimethyltubocurarine. According to many websites, metocurine and dimethyltubocurarine are the same thing. However, metocurine has 3 more methyl groups than normal tubocurarine, not 2, as the other name implies.
Metocurine is sometimes incorrectly called dimethyltubocurarine because when scientists first proposed the structure for d-tubocurarine, they believed it had one more methyl group than it actually did. Metocurine was developed shortly after, before they knew the correct structure of dTC, and going off the incorrect structure, metocurine had two more methyl groups that dTC, hence the name dimethyltubocurarine. It wasn't until 1970, many years after metocurine came out, that researchers discovered that dTC had one less methyl group than originally thought. This means that metocurine actually has 3 more methyl groups than dTC. So really, it's trimethyltubocurarine. If you're not sure, just call it metocurine.
There is also a very nice article I would like you to read. This guy, Scott Smith, was the one who first got me into NMBs, and his experiment mentioned in a book I read when I was maybe 13 was the first I had ever heard of one. I thought what he did was really cool, so my first impression of curare was not "this horrible deadly poison" but rather one of fascination.
The Lack of Cerebral Effects of d-Tubocurarine
But anyways, d-tubocurarine is awesome, and it's easily one of my favorite NMBs.
Please email me , having strange things happening during sleep , Erikaholloway1990@gmail.com